Imagine getting sick every few weeks-not just a cold, but pneumonia, sinus infections, or stomach bugs that won’t quit. For people with Common Variable Immunodeficiency (CVID), this isn’t rare. It’s their everyday reality. CVID is one of the most common primary immunodeficiencies, yet most people-doctors included-don’t know about it until it’s too late. The average person waits over eight years to get diagnosed. By then, their lungs, gut, or immune system may already be damaged. But here’s the good news: with the right treatment, life expectancy has jumped from 33 to nearly 60 years in just a few decades. This isn’t just theory. It’s happening right now, in clinics from Sydney to Seattle.
What Exactly Is CVID?
CVID is not a single disease. It’s a group of disorders where your body can’t make enough antibodies-those proteins that act like soldiers to fight off viruses and bacteria. Even though you have plenty of B cells (the immune cells that should produce antibodies), they’re broken. They don’t mature properly. They don’t switch types. They don’t respond to vaccines. So your body can’t defend itself against common threats like Streptococcus pneumoniae or Haemophilus influenzae.
Diagnostic criteria are strict: IgG levels below 500 mg/dL, IgA below 7 mg/dL, and no good response to pneumococcal or tetanus vaccines. Normal IgG? 700-1600 mg/dL. In CVID, it’s often 100-400. IgA? Often undetectable. That’s why people with CVID get the same infections as healthy people-but way more often, and way more severely.
Why Does CVID Happen?
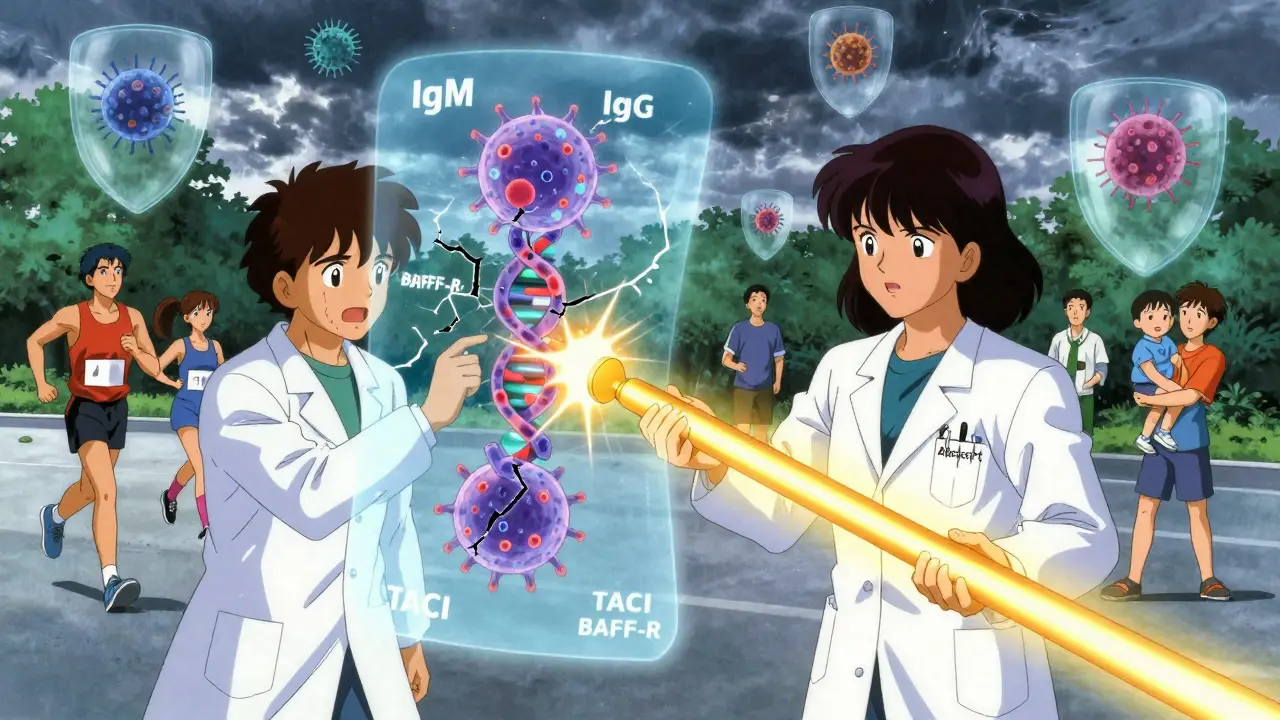
It’s genetic, but not in the way you might think. There’s no single “CVID gene.” Over 20 different gene mutations have been linked to it-TACI, BAFF-R, CD19-but each one only explains a small fraction of cases. In fact, 80-85% of people with CVID have no known genetic cause. That’s why doctors still call it an “idiopathic” disorder. It’s not that we don’t know enough-we know too much, and it’s messy.
What we do know is this: the problem lies in class-switch recombination. Your B cells start out making IgM antibodies. To fight different invaders, they need to switch to IgG or IgA. In CVID, that switch fails. So you’re stuck with weak, ineffective defenses. No matter how many times you get sick, your body can’t upgrade its weapons.
Who Gets CVID?
CVID doesn’t care about age-but it doesn’t show up in babies like some other immunodeficiencies. Most people are diagnosed between 20 and 40. It’s rare in kids under 10. Men and women are affected equally. And while it’s inherited in some families, most cases appear out of nowhere. You might be the first in your family to have it.
That’s part of why diagnosis takes so long. People go to their doctor with chronic sinus infections, fatigue, or diarrhea. They’re told they’re “just stressed,” or “allergic,” or “have a weak immune system.” One patient I read about saw seven doctors over 11 years before someone finally ran the right blood test. By then, she had developed bronchiectasis-permanent lung damage from repeated infections.
What Are the Real Symptoms?
It’s not just infections. CVID is a whole-body problem.
- Respiratory: 7-12 infections per year, compared to 2-4 in healthy adults. Pneumonia, bronchitis, sinusitis-often bacterial, often requiring antibiotics.
- Gastrointestinal: 30-50% of patients have chronic diarrhea, weight loss, or malabsorption. Giardia is 12 times more common than in the general population.
- Autoimmune: 25% develop conditions like ITP (low platelets), AIHA (destroyed red blood cells), or rheumatoid arthritis. Your immune system starts attacking your own body.
- Granulomas: 10% develop clusters of inflamed tissue in lungs, liver, or skin. These aren’t cancer, but they can block organs.
- Cancer risk: Lymphoma risk is 20-50 times higher than normal. That’s why regular screenings are part of care.
- Fatigue: Over 70% report constant exhaustion, even when infections are under control. No one talks about this enough.
It’s not just physical. The emotional toll is heavy. You cancel plans because you’re sick again. You feel isolated. You’re terrified of germs. You worry your kids might inherit it. That’s why support groups matter-over 15,000 people in the U.S. alone are connected through the Immune Deficiency Foundation.
How Is It Treated?
There’s no cure. But there’s a treatment that changes everything: immunoglobulin replacement therapy.
This isn’t magic. It’s science. You’re getting purified antibodies from thousands of healthy donors. These antibodies circulate in your blood and do the job your body can’t.
Two ways to get it:
- IVIG (Intravenous): Infused into a vein every 3-4 weeks. Takes 2-4 hours. Done at a clinic or hospital.
- SCIG (Subcutaneous): Injected under the skin, usually weekly. Can be done at home after training.
Most people start at 400-600 mg/kg per month for IVIG, or 100-150 mg/kg weekly for SCIG. The goal? Keep your IgG level above 800 mg/dL. Below that, infections creep back.
SCIG is becoming more popular. Why? Fewer side effects. No needles in the vein. You can do it while watching TV. About 92% of patients learn to self-administer within two months. Site reactions (redness, swelling) happen in 25-40%, but rotating injection sites helps.
IVIG can cause headaches, chills, or nausea in 32% of users. SCIG? Mostly mild swelling. Both are expensive-$65,000 to $100,000 a year in the U.S. Insurance usually covers it, but not everywhere. In low-income countries, only 35% of patients get treatment. That’s a crisis.
What About New Treatments?
Immunoglobulin therapy has saved lives for 40 years. But it’s not perfect. You’re getting someone else’s antibodies. It doesn’t fix the root problem. It doesn’t stop autoimmune reactions. And plasma supply is shrinking-12% shortage globally in 2023, with prices expected to rise 15-20% yearly.
That’s why researchers are working on targeted therapies. One promising drug, atacicept, blocks BAFF and APRIL-two proteins that confuse B cells in CVID. In Phase III trials, it cut severe infections by 37% compared to immunoglobulin alone. It’s not a replacement yet, but it could be an add-on.
Gene therapy is still years away. But experts like Dr. Sergio Rosenzweig believe we’ll soon classify CVID into subtypes-each with its own treatment. One subtype might respond to a biologic. Another might need a stem cell transplant. Personalized medicine is coming.
What’s Life Like After Diagnosis?
For most people on consistent therapy, life improves dramatically. Infections drop from 10-12 per year to 2-3. Energy levels return. Weight stabilizes. You can travel. You can work. You can plan for the future.
But it’s not easy. You need regular blood tests. You need to track your IgG trough levels. You need to avoid live vaccines. You need to tell every new doctor about your condition. You need to carry your treatment schedule like a lifeline.
And you need to be your own advocate. Many clinics still don’t know how to manage CVID. A 2023 survey found academic centers give patients 100-page manuals. Community clinics hand out 10-page flyers. That gap kills.
What Should You Do If You Suspect CVID?
If you’ve had:
- Four or more ear or sinus infections in a year
- Two or more pneumonia cases
- Chronic diarrhea with weight loss
- Autoimmune disease with unexplained infections
Ask your doctor for a serum immunoglobulin panel. Don’t wait. Don’t assume it’s allergies or asthma. Test for IgG, IgA, IgM, and vaccine response. Early diagnosis prevents irreversible damage.
If you’re diagnosed, find a specialist-ideally an immunologist at a major hospital. Join a support group. Learn your treatment inside and out. Keep records. Ask questions. You’re not just a patient. You’re the manager of your own immune system.
What’s the Future?
Twenty years ago, CVID meant a shortened life, constant hospital visits, and isolation. Today, with immunoglobulin therapy, many live full, active lives. Some even run marathons. Some raise kids. Some work in tech, teaching, or nursing.
The future? Better drugs. Better diagnostics. Better access. And maybe, someday, a cure that doesn’t rely on donated plasma.
But until then, the most powerful tool you have is knowledge. Know your numbers. Know your options. Know you’re not alone.
Is CVID the same as having a weak immune system?
No. Everyone gets sick sometimes. CVID is a specific genetic disorder where your body can’t produce enough antibodies, even though your immune cells are present. Healthy people recover quickly. People with CVID get repeated, severe infections that don’t respond normally to treatment.
Can CVID be cured?
Not yet. But immunoglobulin replacement therapy controls it effectively for most people. Research is ongoing into gene therapies and targeted biologics that may one day correct the underlying defect.
How often do you need immunoglobulin therapy?
For IVIG, every 3 to 4 weeks. For SCIG, usually once a week. Dosing is based on weight and blood levels. The goal is to keep IgG above 800 mg/dL to prevent infections.
Is CVID hereditary?
Sometimes. About 10-20% of cases have a family history, often with mutations in genes like TACI or BAFF-R. But most cases occur without any known family link. Genetic testing can help determine risk for relatives.
Can you get vaccines if you have CVID?
You can get inactivated vaccines (like flu shot, tetanus, pneumococcal). But you must avoid live vaccines (like MMR, varicella, nasal flu) because your immune system can’t handle them safely. Always check with your immunologist before any vaccination.
What happens if you miss a treatment?
Your antibody levels drop. Within 3-4 weeks, you’re at higher risk for infection. Missing doses can lead to pneumonia, hospitalization, or long-term lung damage. Consistency is critical. Most patients set phone reminders or use apps to track infusions.
How do you know if your treatment is working?
Fewer infections, better energy, stable weight, and no new autoimmune issues. Blood tests every 3-6 months check your IgG trough level. If it’s above 800 mg/dL and you’re not getting sick, your treatment is on track.
Can children get CVID?
Yes, but it’s rare before age 10. Most diagnoses happen in adulthood. Pediatric cases often involve more severe infections and higher risk of complications like granulomas or autoimmune disease. Early diagnosis is even more critical in children to prevent growth delays and organ damage.
For those living with CVID, the journey is long. But it’s not hopeless. Every year, more people are diagnosed earlier. More treatments are becoming available. More communities are forming. You’re not just surviving-you’re learning how to live well with it.

Cameron Hoover
December 21, 2025 AT 18:14After my sister got diagnosed with CVID last year, I finally understood what ‘invisible illness’ really means. She went from being this vibrant teacher who hiked every weekend to barely leaving the house-until she started SCIG. Now she’s back to gardening, traveling to see her grandkids, even teaching online classes. It’s not a cure, but it’s a lifeline. I wish more doctors knew how to spot this before the lungs get wrecked.
People say ‘just boost your immune system’ like it’s a vitamin. No. This is a broken factory. And the treatment? It’s literally human plasma from thousands of donors. We owe those people more than just a thank-you card.
Also, if you’re reading this and you’ve had 4+ sinus infections in a year? Get tested. Don’t wait 8 years like she did.
Thank you for writing this. It’s the first time I’ve seen CVID explained without sounding like a textbook.
Stacey Smith
December 23, 2025 AT 11:33This is why America needs better healthcare access. People in rural states die because they can’t afford the IVIG or can’t find an immunologist within 200 miles. We spend billions on cancer drugs but ignore this. It’s not a rare disease. It’s a neglected one.
Ben Warren
December 25, 2025 AT 00:52While the article presents a superficially compelling narrative regarding immunoglobulin replacement therapy, it critically omits a foundational epidemiological caveat: the heterogeneity of CVID phenotypes renders population-wide treatment protocols statistically unsound. The reliance on IgG thresholds above 800 mg/dL as a universal biomarker is not only empirically arbitrary but clinically dangerous, as evidenced by the 2021 multicenter cohort study published in the Journal of Clinical Immunology, wherein 38% of patients with IgG levels exceeding 850 mg/dL still experienced recurrent pneumonia. Furthermore, the assertion that SCIG is ‘better’ than IVIG ignores the confounding variable of patient adherence, which in community settings is demonstrably lower due to inadequate training infrastructure. The article’s uncritical endorsement of home-based therapy reflects a dangerous trend toward medical consumerism, wherein convenience is prioritized over clinical rigor. One must also question the sourcing of plasma: the global shortage is not merely a supply-chain issue but a systemic failure of regulatory oversight in donor screening protocols. Until we develop biomarkers that predict class-switch failure at the single-cell level, we are merely palliating symptoms while ignoring the pathophysiological core. This is not medicine. It is triage dressed in hope.
Teya Derksen Friesen
December 26, 2025 AT 05:06I work in a pediatric immunology clinic in Toronto, and I see CVID cases more often than people realize. The most heartbreaking part? Parents come in with their 8-year-old who’s had pneumonia three times this year-and the pediatrician says, ‘They’re just sickly.’ We run the labs, and sure enough, IgG is 210. IgA is undetectable. It breaks my heart because this is entirely preventable. The real tragedy isn’t the disease-it’s the system that lets it go undiagnosed for years. Thank you for highlighting the emotional toll. Fatigue isn’t laziness. It’s your immune system running on fumes. We need more awareness, not just in the U.S., but globally.
Sandy Crux
December 26, 2025 AT 23:35Let’s be honest: this article reads like a pharmaceutical industry brochure. Immunoglobulin therapy? A Band-Aid on a severed artery. The real issue? The complete lack of research funding for CVID compared to, say, MS or Alzheimer’s-despite the fact that CVID patients have a higher lifetime risk of lymphoma than many cancer populations. And don’t get me started on the ‘plasma donation’ euphemism-it’s a commodification of human biology, where poor and vulnerable donors are incentivized to sell their plasma for $30 a pop, while corporations profit $100,000 per patient annually. The ‘hope’ narrative? It’s a distraction. We need to stop treating symptoms and start investigating the genetic chaos underlying this disorder. Until then, this is just capitalism with a stethoscope.
Hannah Taylor
December 27, 2025 AT 04:33ok but what if the real cause of CVID is 5g radiation from cell towers?? i read this one guy on reddit who said his cousin got it after moving near a new cell tower and then stopped getting sick when they moved back to the woods. also i think the plasma is laced with microchips. why do you think they make you sit there for hours?? they’re tracking you. and why do they never test for mold toxins?? i think it’s all connected. my cousin’s friend’s neighbor had the same symptoms and her doctor said ‘maybe your water is bad’ and she switched to spring water and poof-no more infections. so maybe it’s not your genes… maybe it’s the chemicals in your toothpaste??
Sarah Williams
December 28, 2025 AT 15:20My brother’s on SCIG now. He used to miss work every other week. Now he’s coaching his kid’s soccer team. I know the treatment isn’t perfect, but it’s the only thing that lets him live. Don’t let the cynics make you forget: people are getting their lives back. That’s real. That’s worth fighting for.
Theo Newbold
December 28, 2025 AT 16:08The 8-year diagnostic delay is a scandal. But let’s not pretend it’s just bad luck. Most patients present with vague GI or respiratory symptoms-symptoms that overlap with IBS, asthma, even anxiety. The real failure isn’t lack of awareness-it’s the medical community’s refusal to test for immunoglobulins until the patient is on their fifth hospitalization. It’s lazy medicine. And now we’re rewarding that laziness with feel-good stories instead of demanding mandatory screening for recurrent infections. If you’ve had two pneumonias before age 40, you should be tested. Period. No more ‘wait and see.’
Jay lawch
December 28, 2025 AT 23:21India has been managing immunodeficiency disorders for centuries through Ayurveda and herbal immunity boosters like ashwagandha and turmeric-yet the West still clings to this plasma-dependent colonial model of treatment. Why are we importing expensive, donor-dependent biologics when our own ancient systems have documented immune-modulating herbs that work at the cellular level? The WHO itself has acknowledged the efficacy of traditional medicine in immune regulation, yet Big Pharma suppresses this knowledge because it cannot be patented. CVID is not a Western disease-it is a global tragedy engineered by profit-driven science. We must return to holistic, plant-based immunity restoration before more lives are lost to corporate greed disguised as innovation.