Generic drugs save patients and the healthcare system billions every year. In the U.S., 90.7% of all prescriptions filled are for generics - yet only 23% of total drug spending goes toward them. That’s the power of competition. But behind those numbers is a quiet, critical job pharmacists do every day: spotting when a generic drug isn’t doing what it’s supposed to.
Most generics work just fine. They contain the same active ingredient, same dose, same form as the brand-name version. The FDA requires them to be bioequivalent - meaning the body absorbs them within 80% to 125% of the brand’s rate. That’s a 45% window. Sounds wide, right? For most drugs, it’s fine. But for some, that small variation can mean the difference between healing and harm.
When a Generic Isn’t Just a Generic
Not all generics are created equal. The FDA’s Orange Book rates drugs by therapeutic equivalence. Most get an AB rating - meaning they’re interchangeable. But about 10.3% of generic drugs are flagged as BX. That’s the red flag. These aren’t proven equivalent. They might have different release patterns, different inactive ingredients, or inconsistent dissolution profiles. Pharmacists need to know these codes inside and out.
Take diltiazem CD, a long-acting blood pressure medication. In early 2022, the FDA issued a warning after 47 cases of therapeutic failure were linked to specific generic versions. Patients’ blood pressure spiked because the tablet didn’t release the drug the same way as the brand. The patient didn’t know. The doctor didn’t know. But the pharmacist - the one who handed out the pill - had the data, the training, and the responsibility to notice.
Therapeutic Failure: The Silent Red Flag
Pharmacists should flag a generic when a patient reports something’s off within 2 to 4 weeks after switching. That’s the window where changes show up. A patient says, “My seizures are worse.” Or, “I feel dizzy all day.” Or, “My thyroid numbers went haywire.” These aren’t just complaints. They’re clinical signals.
Narrow therapeutic index (NTI) drugs are the biggest concern. These are medications where even a small change in blood level can cause serious harm. The FDA lists 18 of them. Levothyroxine. Warfarin. Phenytoin. Tacrolimus. Digoxin. For these, a 20% difference in absorption isn’t theoretical - it’s life-threatening.
A 2021 study in the Journal of the American Pharmacists Association found NTI drugs had 2.3 times more therapeutic failures when patients switched between generic manufacturers. One case from the Pharmacy Technician subreddit tells the story: a patient switched generic levothyroxine brands. Six weeks later, their TSH jumped from 2.1 to 8.7 - a clear sign of under-treatment. The pharmacist had to call the doctor, get the original brand back, and monitor closely. That’s not routine. That’s intervention.
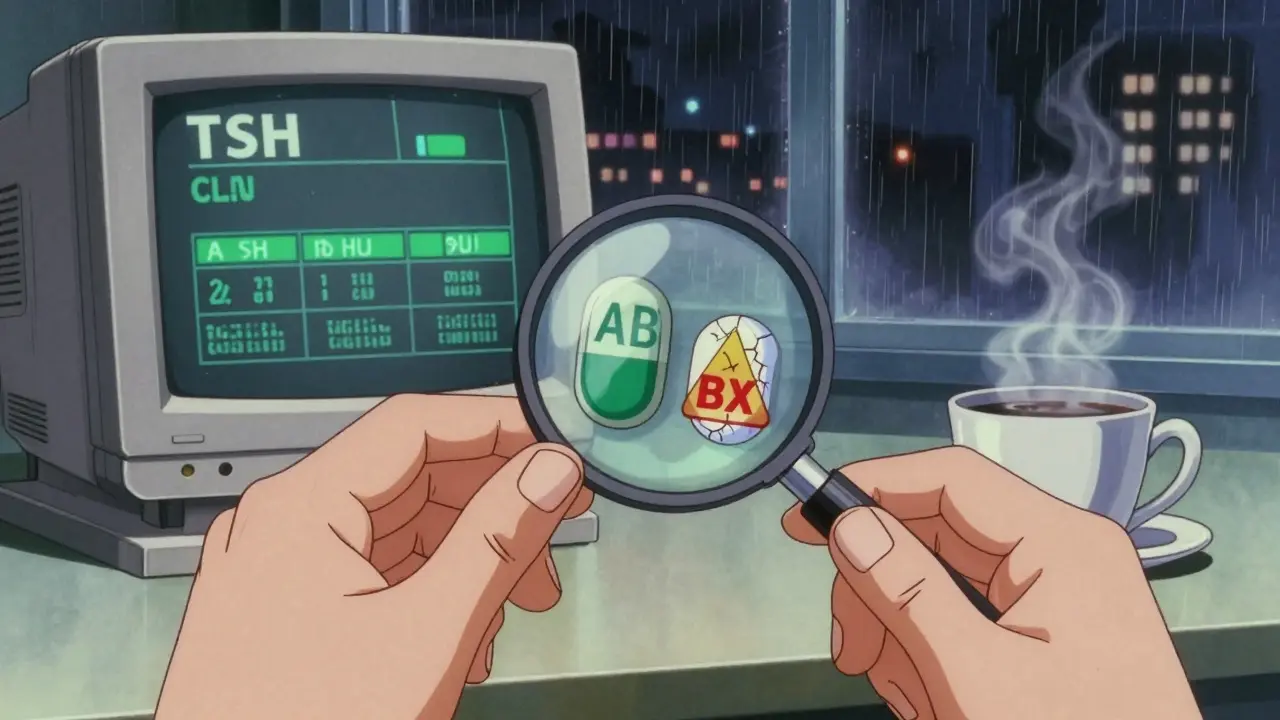
Look-Alike, Sound-Alike: The Hidden Danger
It’s not always about chemistry. Sometimes, it’s about confusion.
Hydrocodone/acetaminophen. Oxycodone/acetaminophen. The names are almost identical. The pills look similar. A busy pharmacy can mix them up - especially when two different generic manufacturers use the same color pill for different drugs. The Institute for Safe Medication Practices reports this accounts for 14.3% of all generic medication errors.
One pharmacist in Ohio told a story: a patient came in for oxycodone/acetaminophen 5/325. They got hydrocodone/acetaminophen 5/325 instead. The patient didn’t know the difference. The doctor didn’t know. But the pharmacist noticed the prescription didn’t match the bottle label. That’s when you stop. That’s when you call.
Complex Formulations: Where Generics Get Tricky
Not all drugs are simple tablets. Extended-release pills, inhalers, patches, injectables - these are harder to copy. The FDA found that 7.2% of generic extended-release opioids failed dissolution testing in 2020. That’s over seven times higher than immediate-release versions. Why? Because the mechanism that controls how slowly the drug releases is complex. Even a tiny change in the coating or granule size can make it release too fast - or not at all.
Patients on extended-release pain meds who suddenly feel sick after a switch? That’s not just “bad luck.” That’s a red flag. Same with inhalers. If a COPD patient starts wheezing more after switching to a generic version, the pharmacist should suspect delivery issues. The drug might be chemically identical, but if it doesn’t reach the lungs the same way, it won’t work.

What Pharmacists Should Do
Here’s the practical checklist:
- Check the Orange Book before dispensing. Look for BX ratings. If it’s there, don’t substitute without consulting the prescriber.
- Document the manufacturer every time. If a patient has an issue, you need to know which version they took. Sixty-eight percent of therapeutic failure investigations require this data.
- Ask patients after a switch: “Has anything changed since you started this new pill?” Don’t assume they know what’s normal.
- Report adverse events to the FDA’s MedWatch system. It takes under five minutes. You’re not just helping one patient - you’re helping thousands.
- Know your state’s laws. In Massachusetts, New York, Texas, and Virginia, you can’t automatically substitute NTI drugs. In other states, you might be required to. Know the rules.
Why It Matters
Patients trust pharmacies. They assume the pill they’re handed is safe, effective, and consistent. When that trust breaks - because a generic didn’t work - it’s not just a medication error. It’s a breakdown in care.
One in five patients who switch generics report different side effects. One in four pharmacists have seen a patient harmed by a problematic generic. These aren’t rare cases. They’re systemic.
The FDA is trying to fix this. They’re increasing inspections. They’re using AI to scan adverse event reports. They’re testing more samples. But no algorithm replaces a pharmacist who’s paying attention.
Generic drugs are essential. They make care affordable. But affordability shouldn’t come at the cost of safety. Pharmacists are the last line of defense. When a patient says, “This doesn’t feel right,” that’s not a complaint. That’s a signal. And it’s your job to listen.
What should a pharmacist do if a patient reports side effects after switching to a generic?
First, confirm the exact generic manufacturer and lot number. Then, check the FDA’s Orange Book for therapeutic equivalence ratings. If the drug is rated BX or if the patient is on an NTI medication, contact the prescriber immediately. Document the reaction and report it to the FDA’s MedWatch system. Monitor the patient’s lab values if applicable - for example, TSH for levothyroxine or INR for warfarin. Never assume the side effect is unrelated to the switch.
Are all generic drugs safe?
Most are. Over 90% of generics meet FDA standards and work as well as brand-name drugs. But no system is perfect. The 80-125% bioequivalence window allows for meaningful differences in drugs with narrow therapeutic indexes. Additionally, manufacturing quality varies - especially with overseas facilities. The FDA found over 1,200 quality issues at foreign plants in 2022. Pharmacists must stay vigilant, especially with complex formulations like extended-release tablets or inhalers.
What are narrow therapeutic index (NTI) drugs?
NTI drugs have a very small range between a therapeutic dose and a toxic dose. Even a slight change in blood levels can cause treatment failure or serious side effects. Examples include levothyroxine, warfarin, phenytoin, digoxin, and tacrolimus. The FDA requires special caution with these. Many states prohibit automatic substitution without prescriber approval. Pharmacists should avoid switching NTI generics unless absolutely necessary and always monitor patients closely after any change.
Can a generic drug be less effective than the brand name?
Yes, in specific cases. While most generics are bioequivalent, differences in inactive ingredients, manufacturing processes, or dissolution profiles can lead to lower effectiveness - especially with extended-release, topical, or injectable formulations. A 2020 FDA study found 7.2% of extended-release generic opioids failed dissolution testing. In real-world use, patients on these drugs have reported sudden loss of symptom control after switching manufacturers. Pharmacists should consider this possibility when a patient reports a change in response.
How can pharmacists prevent generic-related errors?
Pharmacists can reduce errors by: always checking the Orange Book for AB/BX ratings, documenting the manufacturer and lot number on every prescription, asking patients about changes after a switch, using barcode scanning to catch look-alike/sound-alike mix-ups, and staying updated on FDA safety alerts. Continuing education on generics is required in 32 states - use it. Also, encourage patients to report changes. Their feedback is often the first sign of a problem.
John Watts
February 11, 2026 AT 05:16Man, I wish more people knew how crucial pharmacists are. I had a cousin switch generics for her seizure med and almost ended up in the ER. No one told her to watch for changes. Pharmacists aren't just pill counters-they're frontline safety nets. We need to stop treating them like clerks and start respecting them as clinicians.
And yeah, the FDA’s 80-125% window? That’s insane for some drugs. Imagine if your insulin dose varied that much. You’d be dead. But somehow, we let it slide because it’s "cheaper."
Camille Hall
February 11, 2026 AT 18:07This is so true. I work in a community pharmacy, and I’ve had patients come in saying, "I feel weird since I got my refill." Most doctors just shrug. But if you dig into the manufacturer and check the Orange Book, you’ll often find it’s a BX-rated generic. It’s not drama-it’s data.
And documenting the lot number? Non-negotiable. We had a case last year where three patients had the same reaction-all from the same batch. Without records, we’d never have connected the dots.
Ritteka Goyal
February 13, 2026 AT 09:04Ohhh this is sooo true!! I mean like, in India we have like 80% of meds as generics and they work FINE!! Why are Americans so dramatic?? We have cheap, effective medicine everywhere!! Maybe you guys just overthink everything?? Like my uncle takes generic warfarin for 10 years and he's fine!! Maybe you just need to stop being so scared of everything??
Also, I think FDA is just being too strict!! They should let more generics in!! More competition = cheaper!! Why do you need to check every single lot number?? That's sooo slow!! In India we just give the medicine and patients are happy!!
Monica Warnick
February 14, 2026 AT 19:17Okay but like… have you ever seen the packaging on some of these generics? I swear, one time I got a pill that looked like a M&M. I thought I was in a candy store. And then I realized-oh, this is supposed to be my thyroid med. That’s not a pill, that’s a liability waiting to happen.
And don’t even get me started on the smell. Some generics smell like burnt plastic. I swear, I’ve smelled the same generic three times and each time it smelled like a different chemical. I’m not crazy. I have a nose. And I’m not taking it anymore.
Chelsea Deflyss
February 15, 2026 AT 04:20Ugh. I can't believe people still think generics are "just as good." My sister took a generic for her epilepsy and had a seizure in front of her kids. The pharmacist didn't even check the brand. She's lucky she's alive. If you're not checking the Orange Book, you're not doing your job. And if you're substituting NTI drugs without consent? That's malpractice. End of story.
Tricia O'Sullivan
February 16, 2026 AT 14:12Thank you for this thoughtful and meticulously detailed exposition. The systemic underappreciation of the pharmacist’s clinical role is indeed a matter of profound concern. The data you cite, particularly regarding dissolution variability in extended-release formulations, aligns with recent European pharmacovigilance reports. It is not merely a question of cost-efficiency but of pharmacological integrity.
I would respectfully add that international harmonization of manufacturing standards, particularly for APIs sourced from regions with inconsistent regulatory oversight, remains an urgent priority for global patient safety.
Brandon Osborne
February 17, 2026 AT 03:05THIS IS WHY AMERICA IS FALLING APART. You think your pharmacy is helping you? NO. They’re just handing out pills like candy. I had a guy give me a generic for my blood pressure and I ended up in the hospital with a heart attack. The pharmacist didn’t even know the difference between AB and BX. He was scrolling TikTok while filling my script.
And don’t get me started on the FDA. They’re corrupt. They approve generics from factories in China that don’t even have running water. I’ve seen the videos. People are dying because we’re too cheap to care. This isn’t healthcare. It’s a lottery. And I’m tired of losing.
Lyle Whyatt
February 18, 2026 AT 23:00Just wanted to throw this out there-I’m a pharmacist in Sydney, and we’ve had similar issues with generics, especially with anticoagulants and epilepsy meds. We don’t automatically substitute NTI drugs here unless the prescriber explicitly approves it. It’s not about being stubborn-it’s about being responsible.
Also, the look-alike/sound-alike thing? Yeah, it’s wild. We had a case where someone got hydrocodone instead of oxycodone because the bottles looked identical. Patient was fine, but we caught it because the barcode didn’t match. Techs are our heroes. We need more automation and less reliance on human eyes. And yes, I’m totally advocating for color-coded labels. No more beige pills for different drugs. It’s 2025.
Ken Cooper
February 19, 2026 AT 02:18So I’ve been a pharmacy tech for 12 years, and let me tell you-this post is spot on. We document every lot number. We check the Orange Book. We ask patients: "Did anything change?"-and yeah, sometimes they say "no," but then they’ll say it two weeks later: "Oh, I forgot to mention-I’ve been dizzy since last month."
And the FDA’s 80-125% window? It’s not a flaw-it’s a loophole. For drugs like levothyroxine, even 10% difference can throw someone into hyper- or hypothyroidism. I’ve seen TSH levels jump from 3 to 11 just because of a manufacturer switch. It’s not "side effects." It’s biochemistry. And we’re the only ones who catch it.
Also, pls stop saying "all generics are fine." No. Most are. But not all. And the ones that aren’t? They’re the ones that kill people. And nobody talks about it. Until it’s their mom. Or their kid. Or you.